x
Research and Development
-
Kashiv BioSciences > Research and Development
Innovation is a team effort. We believe our people, partners, and shared purpose fuel our research into advancing patient care. Our vertically-integrated approach and state-of-the-art facilities in the United States and India together provide us the means to execute this mission with focus and a deep attention to detail in adhering to the strictest quality standards.

We ground our ambitious R&D efforts in a rigorous selection process to build a diverse, yet thoughtful pipeline of medicines across our key focus areas of biosimilars, complex assets, and mRNA vaccines & therapeutics. Our people, infrastructure, and in-house technology platforms enable us to creatively identify value for patients and partners, and execute across various asset types, regulatory pathways, and therapeutic areas.

Biosimilars
We develop biosimilars with a strong emphasis on quality. Our validated biologics platform is capable of developing and manufacturing monoclonal antibodies (mAbs), fusion proteins, enzymes, antibody-drug conjugates (ADCs), and more. Our approach to pipeline selection and asset development involves a detailed review of the IP landscape to enable speed to market and advantageous competitive positioning.
The Benefit of Biosimilars
Biologic medicines offer important treatment options for life-threatening and disabling diseases. Biosimilars are biologic medicines that have been deemed to be highly similar in quality, safety, and effectiveness to an existing biologic reference product. No clinically meaningful differences exist between the biosimilar and the original product. By introducing competition into the biologic market where there are few low-cost alternatives, biosimilars can contribute to increased, more affordable options for patients.1
Increased Patient Access
The introduction of biosimilars in Europe has resulted in the increased use of biologic drugs in the EU by as much as 100%.2 We anticipate increased usage in the U.S. as more biosimilars become commercially available.
Potential Cost Savings
Biosimilars are priced at substantial discounts versus the innovator biologic products and are expected to provide cost savings of up to $150 billion in the U.S. alone by 2026.3,4
Complex Programs
Our R&D approach is predicated on leveraging our unique and highly refined capability set to develop complex, difficult-to- formulate assets utilizing ANDA or 505(b)(2) pathways. We vary our approaches to best serve unmet patient needs in conjunction with a disciplined approach to risk management and IP strategy.
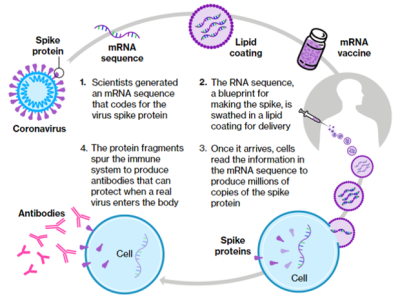
mRNA Therapies
Kashiv is developing an mRNA platform initially focused on prophylactic mRNA vaccine development, including a second- generation COVID-19 vaccine. Our platform technology is powered by in-house LNP and capping enzyme capabilities.
References :
1 “Biosimilars: More Treatment Options Are on the Way,” FDA Consumer Health Information, 2016.
2 “Delivering on the Potential of Biosimilar Medicines,” IMS Institute, 2016.
3 “Biosimilars: Infancy to Youth – Outlook Through 2025,” Morgan Stanley Research Global Insight, 2019.
4 “Biosimilar Cost Savings in the United States: Initial Experience and Future Potential,” Rand Health Quarterly, 2018.
2 “Delivering on the Potential of Biosimilar Medicines,” IMS Institute, 2016.
3 “Biosimilars: Infancy to Youth – Outlook Through 2025,” Morgan Stanley Research Global Insight, 2019.
4 “Biosimilar Cost Savings in the United States: Initial Experience and Future Potential,” Rand Health Quarterly, 2018.
